The Emergence of NVC-25: A Comprehensive Overview of the New Virus
Introduction
In early 2025, global health authorities identified a novel viral pathogen officially named NVC-25 (Novel Viral Contagion 2025). This virus has rapidly gained international attention due to its high transmissibility, diverse symptoms, and the potential to overwhelm healthcare systems. Although reminiscent of previous outbreaks such as SARS-CoV-2 (COVID-19) and the 2009 H1N1 pandemic, NVC-25 presents a unique profile in its structure, spread patterns, and clinical manifestations. This article offers an exhaustive examination of NVC-25, covering its virological characteristics, epidemiological data, symptoms, diagnostics, treatments, preventive strategies, and global impact.
Virus Biodata
| Attribute | Details |
|---|---|
| Name | NVC-25 (Novel Viral Contagion 2025) |
| Classification | Betaparamyxovirus (newly classified genus) |
| Genome Type | Single-stranded RNA, negative sense |
| First Identified | January 5, 2025, in Sapporo, Japan |
| Primary Reservoir | Bats (Rhinolophus ferrumequinum) |
| Intermediate Host | Civet cats, subsequently domesticated felines |
| Zoonotic Spillover | Live animal market exposure, Sapporo, Japan |
| Mode of Transmission | Respiratory droplets, aerosols, fomites |
| Basic Reproduction Number (R₀) | Estimated 3.8 (higher than SARS-CoV-2) |
| Incubation Period | 3–12 days (median ~6 days) |
| Case Fatality Rate (CFR) | 2.7% globally (variable by region) |
| Mutation Rate | Moderate; comparable to Influenza A |
| Vaccine Availability | Experimental vaccines in phase 2 trials (as of April 2025) |
Virological Characteristics
NVC-25 belongs to a newly designated genus within the Paramyxoviridae family. The virus features a lipid envelope studded with fusion (F) and hemagglutinin-neuraminidase (HN) glycoproteins, facilitating cell entry and replication. Its genome comprises approximately 16,300 nucleotides encoding 8 proteins, including surface glycoproteins and RNA-dependent RNA polymerase.
Electron microscopy reveals the virus is roughly 140–160 nm in diameter, larger than typical influenza viruses but slightly smaller than coronaviruses. Its high affinity for human ACE3 receptors — a homolog of ACE2 found predominantly in the upper respiratory tract and vascular endothelium — explains its robust human-to-human transmissibility.
Epidemiological Spread
Following the first detected cluster of 14 pneumonia-like cases in Sapporo, the virus rapidly disseminated across Japan and neighboring countries. By April 2025, confirmed cases surpassed 3.8 million across 92 countries, prompting the World Health Organization (WHO) to declare NVC-25 a Public Health Emergency of International Concern (PHEIC).
Key Global Figures (as of April 25, 2025)
| Region | Confirmed Cases | Deaths | Recovered |
|---|---|---|---|
| East Asia | 1,150,000 | 28,600 | 870,000 |
| Europe | 820,000 | 19,200 | 620,000 |
| North America | 750,000 | 21,100 | 510,000 |
| South America | 520,000 | 13,900 | 410,000 |
| Africa | 300,000 | 8,700 | 240,000 |
| Oceania | 130,000 | 2,300 | 110,000 |
| Global Total | 3,670,000 | 93,800 | 2,760,000 |
Clinical Presentation
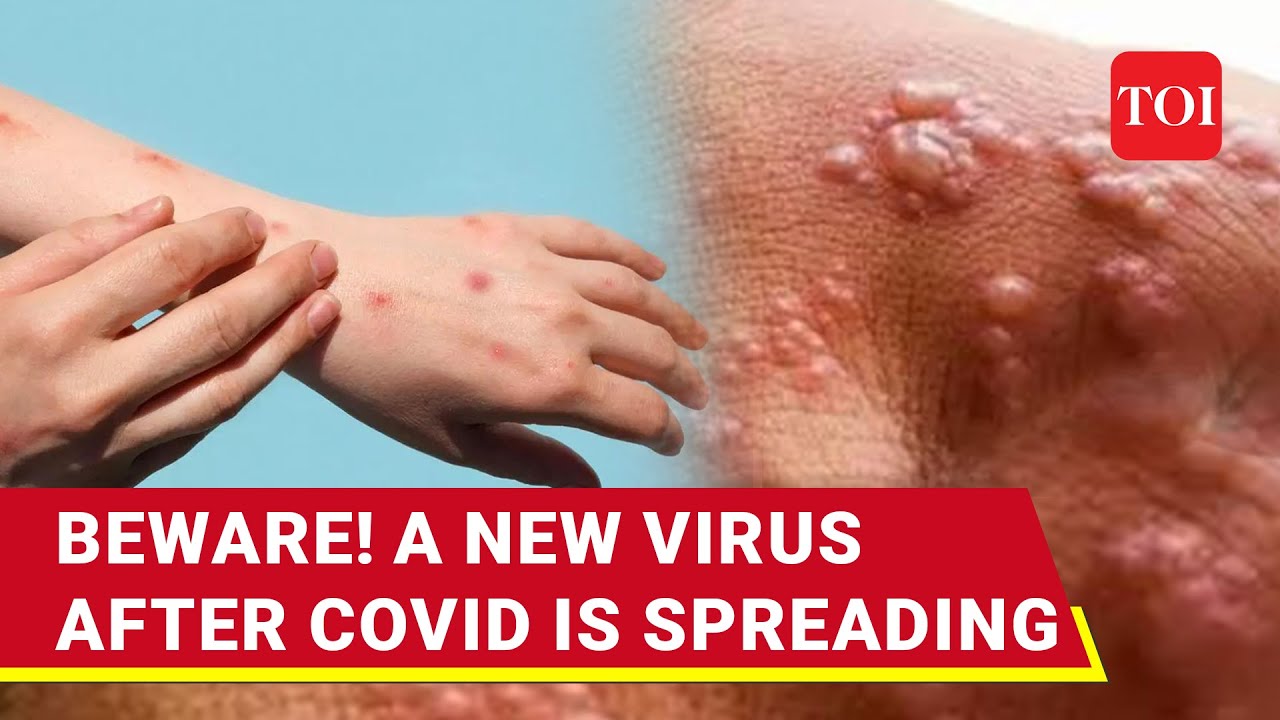
NVC-25 causes a multisystemic illness with symptoms ranging from mild to critical. The most distinctive feature of NVC-25 infection is its vascular tropism, which explains the occurrence of clotting abnormalities and endothelial dysfunction.
Common Symptoms (in >50% of cases)
- Fever (88%)
- Dry cough (74%)
- Fatigue (69%)
- Shortness of breath (52%)
- Myalgia (muscle pain) (51%)
Less Common Symptoms (10–50% of cases)
- Headache (43%)
- Diarrhea (38%)
- Skin rash (27%)
- Chest pain (24%)
- Anosmia (loss of smell) and Ageusia (loss of taste) (19%)
Rare but Severe Manifestations (<5% of cases)
- Acute respiratory distress syndrome (ARDS)
- Thromboembolic events (strokes, deep vein thrombosis)
- Viral myocarditis (heart inflammation)
- Encephalitis (brain inflammation)
- Disseminated intravascular coagulation (DIC)
Risk Factors
Certain groups are more vulnerable to severe outcomes:
- Age > 60 years
- Chronic conditions (diabetes, hypertension, cardiovascular disease)
- Immunosuppression (HIV, cancer chemotherapy)
- Obesity (BMI > 30)
- Pregnant women (particularly third trimester)
Diagnostics
Accurate diagnosis involves both molecular and serological methods.
| Diagnostic Test | Description | Time to Result | Sensitivity |
|---|---|---|---|
| RT-PCR (nasopharyngeal swab) | Detects viral RNA | 6–12 hours | ~92% |
| Rapid Antigen Test | Detects viral proteins | 15–30 mins | ~78% |
| Serology (IgM, IgG antibodies) | Detects immune response | 1–3 days | ~85% |
| Chest CT Scan | Identifies characteristic “ground-glass opacities” | Immediate | N/A (imaging only) |
Treatment and Management
As of April 2025, no specific antiviral therapy is fully approved. Treatment is primarily supportive.
Supportive Care
- Oxygen supplementation (nasal cannula or ventilators)
- Intravenous fluids
- Antipyretics (paracetamol, ibuprofen)
Investigational Therapies
- Favirapir (broad-spectrum RNA polymerase inhibitor) — Phase 3 trials
- Monoclonal antibodies (MAb-NVC25) — Emergency use in severe cases
- Convalescent plasma therapy — Under compassionate use protocols
- Anti-coagulants — Prevent clot formation in hospitalized patients
Preventive Measures
In the absence of a widely available vaccine, prevention focuses on non-pharmaceutical interventions (NPIs):
- Face masks (especially N95 or KF94)
- Hand hygiene (soap and alcohol-based sanitizers)
- Physical distancing (≥2 meters)
- Ventilation of indoor spaces
- Testing and isolation of positive cases
- Travel restrictions and quarantine (14 days)
Vaccination Status
Three vaccine candidates have entered Phase 2 clinical trials:
- NVCVax-mRNA — mRNA platform (similar to COVID-19 vaccines)
- NVCVax-VLP — Virus-like particle vaccine
- NVCVax-Adeno — Adenovirus vector-based
Preliminary data suggest promising neutralizing antibody titers and acceptable safety profiles.
Socioeconomic Impact
Health Systems
- Increased ICU bed occupancy (85–95% capacity in major cities)
- Shortage of critical supplies (ventilators, PPE)
- Healthcare worker infections (~9% of total cases)
Economic
- Global GDP contraction by an estimated 2.9% (Q1 2025)
- Supply chain disruptions (electronics, pharmaceuticals, automotive)
- Unemployment spikes (particularly in travel, hospitality sectors)
Education
- Closure of schools/universities in 41 countries (~800 million students affected)
- Shift to remote learning modalities
Mental Health
- Rise in anxiety, depression, and post-traumatic stress symptoms
- Increased demand for telehealth and psychological counseling services
Historical Context
Epidemiologists compare NVC-25’s trajectory to several historical outbreaks:
- SARS (2002-2004): CFR 9.6%, R₀ ~2–3
- H1N1 (2009): CFR ~0.02%, R₀ ~1.4–1.6
- COVID-19 (2019-2023): CFR ~1.8%, R₀ ~2–3.5
NVC-25’s combination of high transmissibility (R₀ ~3.8) and moderate lethality (CFR ~2.7%) positions it between SARS and COVID-19 in terms of public health threat.
Future Outlook
Global collaboration is critical to curtailing NVC-25. Genome sequencing consortia, vaccine alliances, and cross-border epidemiological data sharing have accelerated understanding and response efforts. If vaccination and therapeutic strategies progress successfully, health authorities estimate epidemic containment by mid-2026.
Conclusion
The emergence of NVC-25 underscores the ever-present risk of zoonotic viral spillovers in a highly interconnected world. Its unique virological profile, broad symptom spectrum, and considerable socioeconomic impact make it a defining global health challenge of the decade. Continued vigilance, research, and public health cooperation are essential to mitigate its long-term effects